Kātahi ka piki haere te hiahia o te ao ki ngā raihana penapena nui e taea ai te whakamahi i te kaha, ka puta ake ngā taiapa mātātoko (Na-ion) hei kōwhiringa pai ake i ngā tikanga tawhito mō ngā taiapa līthiemi (Li-ion). Mā te nuinga o ngā rawa matua, te iti ake o te pānga ki te taiao, me te mahi paraihe āhuarangi e āki nei ana ngā taiapa Na-ion ki ngā whakamahinga mai i te penapena kaha ki te roopu nui ake, waka inuhi, hoki ki ngā taputapu mō te kaihoko. Kei te pū o tēnei whanaketanga he tikanga paraihe: ko te nuinga o ngā iona natriki e hoki haere ana i waenganui i te cathode me te anode i te wā o te whakakī me te whakangao. I tēnei tuhinga, ka āta tirotiro mātou i ngā tikanga herehere e arataki ana i ngā mōhiotia o te whakakī me te whakangao o ngā taiapa Na-ion, ka ākina i te mōhio kei te tūāpapa pea tēnei hangarau hei huri i te tauira o te penapena kaha i te wā e tū ake nei.
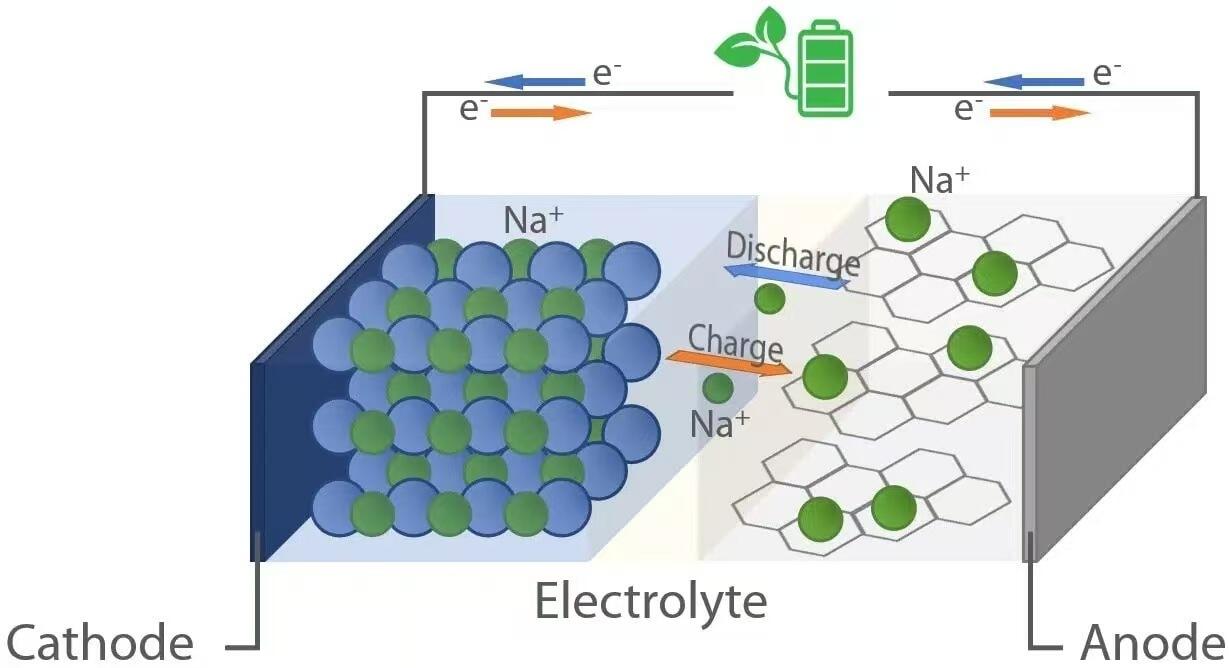
He rite tā ngā riipene taiapa-mata (sodium-ion) ki ō rātou hoa taiapa-lithium, e whakaeke ana i te mātai kemikara “rocking-chair”. I te wā o te whakaputanga—ka hūnuku te taiapa i tētahi taputapu—ka nekeneke ngā ion taiapa (Na⁺) mai i te anode (karawhiti taketake) tru ngā wehenga hiko ki te cathode (karawhiti irahiko). I te wā kotahi, ka rere ngā electron tru te ara a-waho, ka tuku hiko ki te uta hiki. Engari, i te wā o te uta, ka taraiwa e tētahi punaha a-waho ngā ion taiapa mai i te cathode ki te anode, ka whakaputangia te hiko mō ngā whakamahinga āmuri. Ko tēnei kaupapa kaokao hokiropū e āwhina ana i ngā matū kuaamui i ngā karawhiti e taea ai te whakauru (toihana) me te whakangāwari (tango) i ngā ion taiapa kaua ko te pito rerekētanga o te hanganga.
Ina whakamahia te rewharewha mātātoko-mata (sodium-ion) ka pakaru te anōta i te anō. Ko ngā matū e whakamahia ana ko te karbona haka, he tikanga kore pakuhi ā tana rerehua he wāhi iti ka taea te noho mā te Na⁺. Ina tuku kaha te rewharewha, ka wehe ngā atamu sodium i roto i te anō i ngā inihana (e⁻) ka huri ki te Na⁺:
Anō (Whakaputa):
Na → Na⁺ + e⁻
Ka rere ēnei inihana truha i te ara takotoranga ki te taputapu kia ora ai, i te wa e neke ana ngā iona Na⁺ truha i te arai reka, he rorohiko, ki te katō. I te katō—e hangaia mātua e ngā oksīti metara takirua (hei tauira, NaₓMO₂, nona M = Mn, Fe, Ni, ākī), ngā huinga polyanionic, i ngā tauira Prussian blue—ka pā te whakarereketanga ina uru ana ngā iona Na⁺ me ngā inihana ki roto i te rerehua kiriata:
Katō (Whakarereketanga):
Na⁺ + e⁻ + Kaitiaki → Na–Kaitiaki
Ko tēnei whakaurunga e whakapumautia ana te hanganga kaitoi, ā, ka oti te porowhita electrochemical. Ko te tokiwha i whakaputangia i te wā o te tango pūngao e noho ana ki te rerekētanga o te uara electrochemical i waenganui i ngā matēria anode me ngā matēria kaitoi, e 2.5 ki te 3.7 volts ina hokona ngā sel Na-ion.
I te wā o te utu, ka whakamahia tētahi tokiwha waho e nui ake ana i te tokiwha waho o te sel, ka huri ngā urupare electrochemical. Ka tangohia ngā iona sodium mai i te kaitoi mā te oxidization:
Kaitoi (Oxidation):
Na–Host → Na⁺ + e⁻ + Host
Ka hīkoi ngā iona Na⁺ kua whakapaipai ki te haonga anō ki te anode, ā, ka hoki anō ngā electron mā te puna pūngao waho. Ki te anode, ka aru tētahi reduction a ka honoa ngā iona Na⁺ ki ngā electron, ka hoki anō ki roto i te matrix karboni:
Anode (Reduction):
Na⁺ + e⁻ → Na (intercalated)
Whakamahia tēnei tikanga hei whakaora i te kaha o te pūhiko, hei raihana mō te karuwha tukanga e whai ake nei. He mea nui te kounga o te uta kawe, ngā arotake tītaha iti, me te pūmanawa o ngā matapae o ngā taponga hikoira kia eke ki te roa o te karuwha me te tikanga Rukaia o te Kourama—ngā inenga matua mō te āhua herehere.
Ko te hikoira—heoi anō he kōpae natriemi (hei tauira, NaClO₄, NePF₆) kei roto i ngā waiarai karapoti tāpoi—ka takirua he roa nui ki te whakauru tere i ngā ioni, i te wa e manaaki ana i te pūmanawa hikoira. I ngā karuwha tuatahi, ka hangaia he papanga hikoira-kōpae taupiri (SEI) ki te mata o te anōta. Whakarereketia e tēnei papanga whakamarumaru i te wehenga atu anō o te hikoira, i te wā e whakaae ana i ngā ioni Na⁺ ki te toronga atu—he tohatoha māmā noa e hiahiatia ana mō te haumātauranga me te roa o te ora.
Ko te nuinga ake o te rino (neke atu i te 1,000 ngā wā ake i te rino ki te takotoranga o te whenua) ka hua ai he utu iti ake mō ngā matērira, me te whakaiti ake i ngā tūponotanga e pa ana ki te tukunga mai i ngā wāhi motuhake. Anō hoki, ka taea te whakamahi i te karu-aluminium hei kai-kiroiroi mō te anode i roto i ngā pūhiko Na-ion (e kore e taea i ngā pūhiko Li-ion, e hiahiatia ana te paraihe), ka iti ake ai te utu me te taumaha. Heoi anō, he nui ake, he taumaha ake hoki ngā iona rino i ngā iona litium, ka hua ai he iti ake te kotahi o te energiya, me te arataki iti ake i ngā iona. Ko te rangahau e haere nei e hiahia ana ki te whanaketanga i ngā hanganga umanga, ngā matērira hanga-raraunga, me ngā wehenga pūkaupapa kia taea ai te whakawātea i ēnei here.
Ko ngā tikanga utu me te tango i ngā pūhiko mātātoko-matauranga he tauira o te aronga pai ki waenganui i te putanga matū me te kimia hiko, e whakapumautia ana hei takotoranga kaha mō te rokiroki wairua o te whakaeke. Arā rānei i ngā karawhiti-lithium, ko te here ki te sodium e noho nui ana, iti te utu e kore e whakamātau i ngā tūranga tukutuku engari e rite ana hoki ki ngā whāinga ā-ao mō te tūmanako. I te haerenga a ngā kairangahau e whakapai ake nei i ngā huinga o ngā electrore—e whakapai ake ana i te pūmahara me te kotahi o te wairua—whakapai ake i ngā huinga electrolyte kia piki ake te ora mara me te haumaru, me te whakapai ake i ngā tikanga hangarua nui kia iti ake te utu hua, kei te tino pakari haere te hanganga mātātoko-mataurangi i ngā wāhi e totohu ana. Ko te whakapai ake nei e tuana ana i ngā pūhiko Na-ion kia mahi heke whenua i te huringa o ngā punaha wairua ā-motu, mai i te rokiroki taupā e tautoko ana i te whakaurunga o ngā rongoā hiko ki te hiko atamira, ki te hiko pūmau, ki te nukumehina hiko iti-korenga. Mā te whakamahi i te nekeneke anuanu engari hoki kaha o ngā ion mātātoko, kāore i te whakamāori i te hiko anake i te āhua me te utu iti—engari kei te hanga hoki i tētahi kaupapa wairua e wātea ake ana, e pakari ake ana, e tūmatakaria ana. Kei te hono i te āhuatanga o te ao hiko me te whakamahinga o te ao, e tuku ana i tētahi ara e taea ana kia iti ake ngā huakita karbon me te hanga i tētahi ekosiateme wairua ā-whenua kia kākāriki ake.
 Pānui Pāhotanga
Pānui Pāhotanga