As the global demand for sustainable, cost-effective, and high-performance energy storage solutions continues to surge, sodium-ion (Na-ion) battery technology has emerged as a compelling alternative to conventional lithium-ion systems. With abundant sodium resources, lower raw material costs, and promising electrochemical performance, Na-ion batteries are gaining significant traction across electric mobility, grid-scale storage, and consumer electronics. However, the key to unlocking their full potential lies in the intelligent design and selection of cathode and anode materials—two critical components that define energy density, cycle life, safety, and overall efficiency.
Unlike lithium, which readily intercalates into layered oxides like LiCoO₂ or NMC (nickel-manganese-cobalt), sodium’s larger ionic radius presents unique challenges for cathode development. Researchers have therefore explored three primary families of cathode materials for Na-ion batteries: layered transition metal oxides (NaxTMO₂), polyanionic compounds, and Prussian blue analogs (PBAs).
Layered oxides—particularly those based on nickel, manganese, iron, and copper—offer high specific capacities (often exceeding 120 mAh/g) and good rate capability. For instance, O3-type NaNi₁/₃Mn₁/₃Co₁/₃O₂ delivers excellent capacity but suffers from structural instability during deep cycling due to phase transitions. In contrast, P2-type structures (e.g., Na₂/₃Ni₁/₃Mn₂/₃O₂) exhibit better cycling stability and faster Na⁺ diffusion, making them more suitable for long-life applications. Recent advances focus on doping strategies (e.g., Mg²⁺, Ti⁴⁺) and surface coatings to suppress oxygen loss and mitigate volume changes.
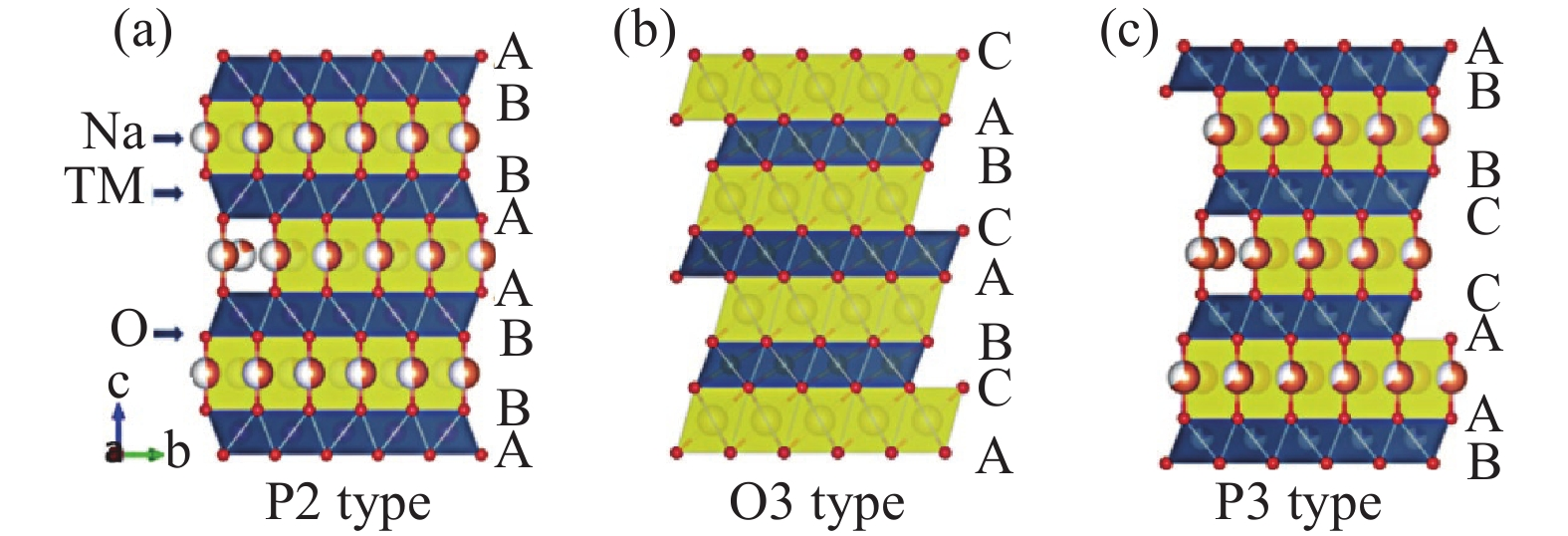
Schematic diagram of layered oxide structure
Polyanionic cathodes, such as Na₃V₂(PO₄)₃ (NVP) and fluorophosphates like NaVPO₄F, provide exceptional thermal and structural stability thanks to their robust covalent frameworks. While their theoretical capacities are modest (~117 mAh/g for NVP), they deliver ultra-long cycle life (>10,000 cycles) and operate at higher voltages (~3.4 V vs. Na⁺/Na). Moreover, vanadium-free alternatives—such as iron-based phosphates—are being developed to reduce toxicity and cost, aligning with sustainability goals.
Prussian blue analogs represent a third frontier. Their open framework allows rapid Na⁺ insertion/extraction, enabling high power density. However, challenges remain in controlling water content within the crystal lattice, which can degrade performance and safety. Innovations in synthesis—such as low-temperature co-precipitation under inert atmospheres—are improving crystallinity and reducing lattice defects, bringing PBAs closer to commercial viability.
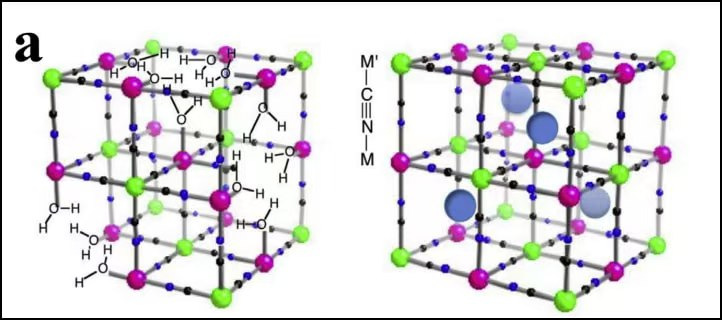
Schematic diagram of the crystal structure of Prussian blue and its derivatives
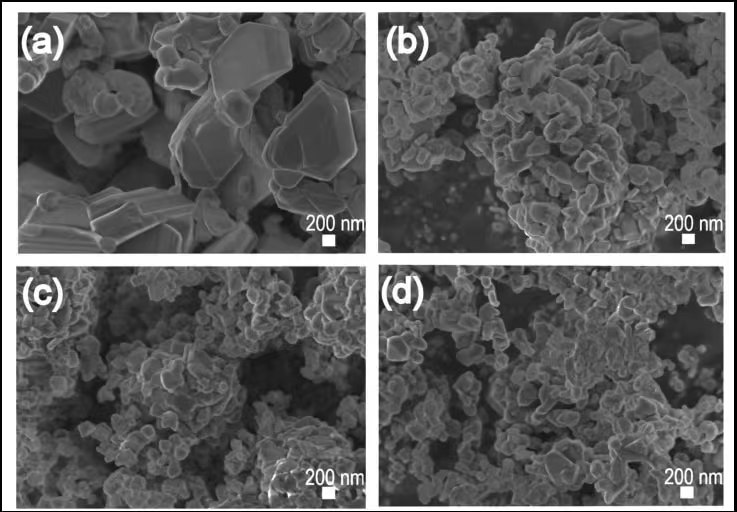
SEM images of Prussian blue and its derivatives
While graphite is the standard anode in lithium-ion batteries, its interlayer spacing (~3.35 Å) is too narrow to accommodate Na⁺ ions efficiently, resulting in negligible capacity. This limitation has spurred intense research into alternative anode materials.
Hard carbon stands out as the most commercially viable option today. Its disordered structure features expanded interlayer spacing (>3.7 Å) and nanopores that facilitate Na⁺ storage via both adsorption and pore-filling mechanisms. Hard carbon anodes typically deliver reversible capacities of 250–320 mAh/g with good initial Coulombic efficiency (>85%). Sourcing precursors sustainably—from biomass (e.g., coconut shells, lignin) or recycled polymers—not only lowers costs but also enhances environmental credentials.
Beyond hard carbon, alloy-based anodes (e.g., Sn, Sb, P) offer extremely high theoretical capacities (e.g., 847 mAh/g for Na₃P). Yet, these materials undergo massive volume expansion (>300%) during sodiation, leading to particle pulverization and rapid capacity fade. Nanostructuring, carbon compositing, and binder engineering are proving effective in mitigating mechanical degradation and improving cyclability.
Another promising avenue involves conversion and intercalation-type materials like titanium-based oxides (e.g., Na₂Ti₃O₇) and MXenes. These exhibit minimal volume change and excellent safety profiles, albeit at the expense of lower capacity and operating voltage. They are particularly attractive for stationary storage where energy density is less critical than longevity and reliability.
The optimal Na-ion battery isn’t defined by a single “best” material but by the synergistic pairing of cathode and anode that balances voltage window, kinetics, and interface compatibility. For example, coupling a P2-type layered oxide cathode with a biomass-derived hard carbon anode enables cells with >140 Wh/kg energy density and >5,000-cycle lifespan—metrics competitive with LFP (lithium iron phosphate) batteries.
Moreover, electrolyte formulation and solid-electrolyte interphase (SEI) engineering play pivotal roles in stabilizing electrode/electrolyte interfaces, especially given sodium’s higher reactivity compared to lithium. Additives like fluoroethylene carbonate (FEC) significantly enhance SEI quality, reducing irreversible capacity loss during initial cycles.
As global supply chains grapple with mounting pressures from lithium and cobalt scarcity, sodium-ion technology emerges as a resilient, geographically diversified alternative that breaks reliance on limited resources. By tailoring material selection to meet application-specific demands—high energy density for electric vehicles, ultra-long cycle life for renewable energy integration, or cost-effectiveness for consumer electronics—sodium-ion batteries are well-positioned to become a cornerstone of the next-generation energy ecosystem, complementing existing storage solutions and unlocking new application scenarios worldwide. This shift not only addresses supply chain vulnerabilities but also aligns with global decarbonization goals, paving the way for a more sustainable energy landscape.
At Zhejiang Mingtu Technology Electrical Co., Ltd., we are dedicated to turning this vision into reality with our core competitive strengths. We lead in cutting-edge R&D of high-performance electrode materials, boasting independent formulas that enhance battery energy density and cycle life. Our optimized scalable manufacturing processes, supported by intelligent production lines, ensure stable quality and cost control for mass production. Moreover, our holistic cell design integrates efficiency, safety, and cost—backed by rigorous testing—to meet diverse industrial demands. The future of energy storage is not merely about replacing lithium; it’s about reimagining possibilities with smarter chemistry, ethically sustainable sourcing, and innovative engineering. As Earth’s sixth-most abundant element, sodium holds immense potential—and we are leveraging its unique advantages, along with our technical prowess, to deliver reliable, accessible energy storage solutions that power a greener, more resilient future for global industries and communities.